Ozonator
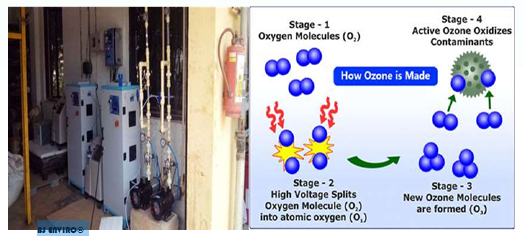
The chemical reaction that results in ozone is pretty simple. Ozone is a form of oxygen that is created when electrical energy breaks apart an ordinary oxygen molecule (O2) starting a chemical reaction that results in ozone (O3).
Electrical energy breaks the ordinary O2 molecule into two O1 atoms The free oxygen at omsunite with other O2 molecules to produce zone (O1) + (O2) =(O3)
Ozone is an unstable molecule because the 3rd oxygen atom is connected to the other two atoms with a weak bond. The weak bond is why ozone is such a powerful sanitizer.
Applications :-
1. Powerful disinfectant to remove Salmonella, Listeria, E. coli or any number of other dangerous microorganisms.
2. Colour less Treated Water.
3. Odour less Treated Water.
4. Suitable even if there is uneven supply ofsewage.
5. Highly suitable for inconsistentsewage.
6. Overall Organic & Inorganic content reduction (BOD&COD),Metalsiron,copper and manganese, and inorganic forms of chloramine &Nutrient.
7. Less Sludge Production.
8. Low Space Requirement.
9. Dose not requires continuous operation.
10. Ozone is highly effective in cold water.
11. MLSS and SVI monitoring not required.
12. Environmentally Safe to treat sewage water disposed in Land or Water.
13. Treated flushing, irrigation, construction and many more potableuses.
14. Environmentally Preferred Sanitizer, leaving behind onlyoxygen.
